|
The Kinetics of Phosphate Radical Ions in Aqueous Solutions (a)
Janina A. Rosso, Mónica C. Gonzalezb and Daniel O. Mártirec
Instituto de Investigaciones Fisicoquímicas Teóricas y Aplicadas (INIFTA), Facultad
de Ciencias Exactas,
Universidad Nacional de La Plata, Casilla de Correo 16, Sucursal 4, (1900) La Plata,
ARGENTINA.
FAX: --54 21 25 4642; e-mail: dmartire@volta.ing.unlp.edu.ar
___________________________________________________________________ a Manuscript
in preparation.
b Research member of Consejo Nacional de Investigaciones
Científicas y Técnicas (Argentina).
c Research member of Comisión de Investigaciones Científicas
de la Provincia de Buenos Aires (Argentina).
Keywords: Phosphate radical ions, flash-photolysis, oxygen adducts.
INTRODUCTION
Phosphate radicals exist in three acid-base forms (PO4H2., PO4H.-, PO4.2-)
related by fast equilibria (eq. (1)). These radicals were reported to react with organic
and inorganic substrates [1] either by hydrogen abstraction or by electron transfer with
different efficiency.
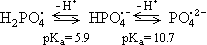 (1) (1)
Huber and Hayon [2a] studied the decay kinetics of phosphate radicals generated by
VUV photolysis of HPO42-
and H2PO4.
(e.g. reaction (2)).
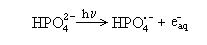 (2) (2)
These authors observed a second order decay of phosphate radicals in Ar-saturated
solutions and a first-order decay kinetics in the presence of molecular oxygen. These
results were interpreted in terms of the formation of oxygen adducts with HPO4.- or H2PO4. [2a]. In all
other more recent literature reports, phosphate radicals were generated in the absence of
molecular oxygen [2b].
Waste-water oxidative procedures based on the generation of HO.
or SO4.- [3]
radicals initiating organic substrates oxidation, may also form phosphate radicals (PO4H2.,
PO4H.-, PO4.2-) from the reaction of HO./
SO4.- with
phosphate ions contained in high concentrations in the water matrix [1, 4]. However, the
information reported in the literature on the reactivity of the phosphate radicals is
scarce [1, 5].
We here studied the decay kinetics of PO4H2., PO4H.-,
PO4.2- generated by photolysis of peroxodiphosphate ions (reaction
(4)) in N2 and O2-saturated
solutions, both in the presence and absence of added substrates.
|
